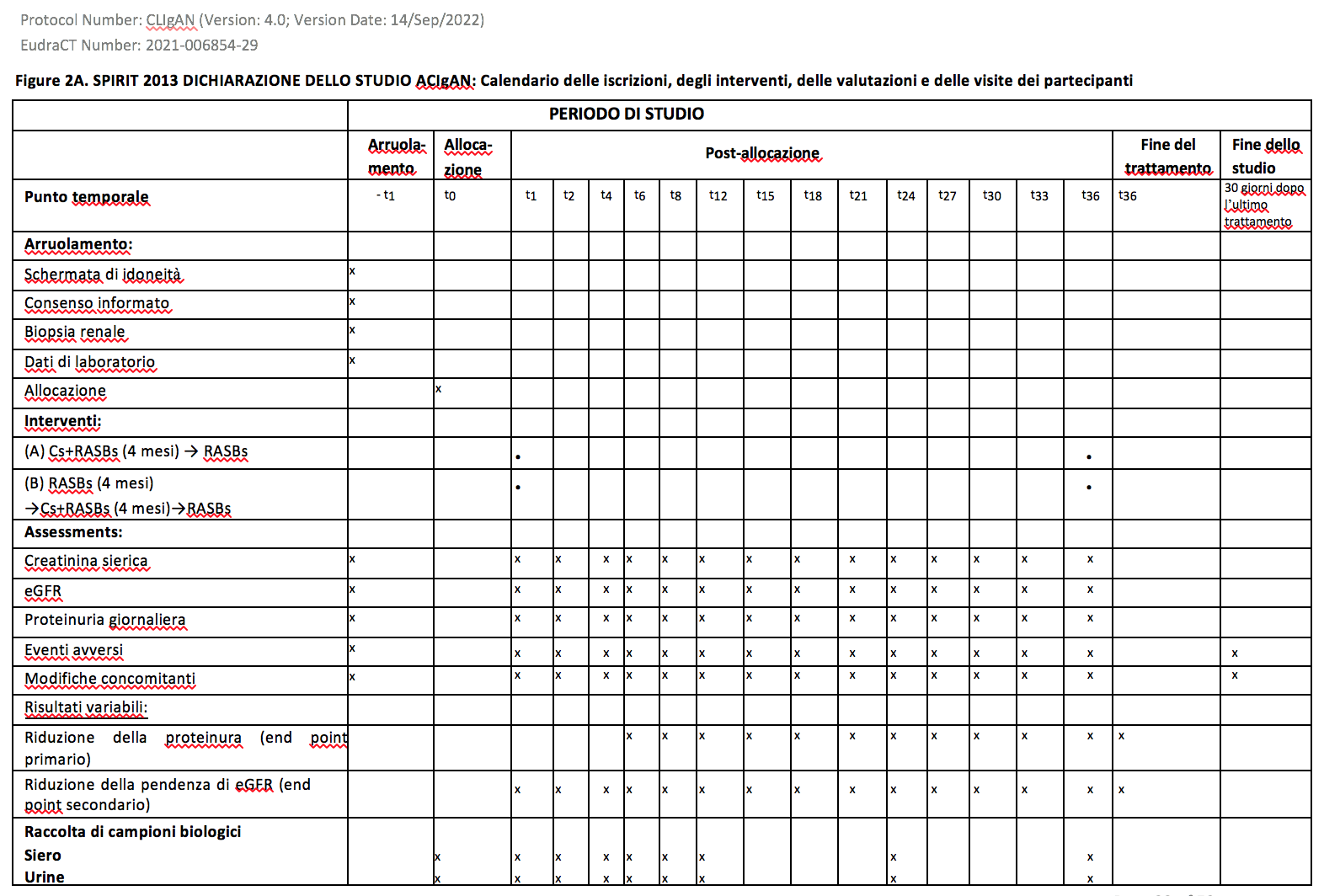
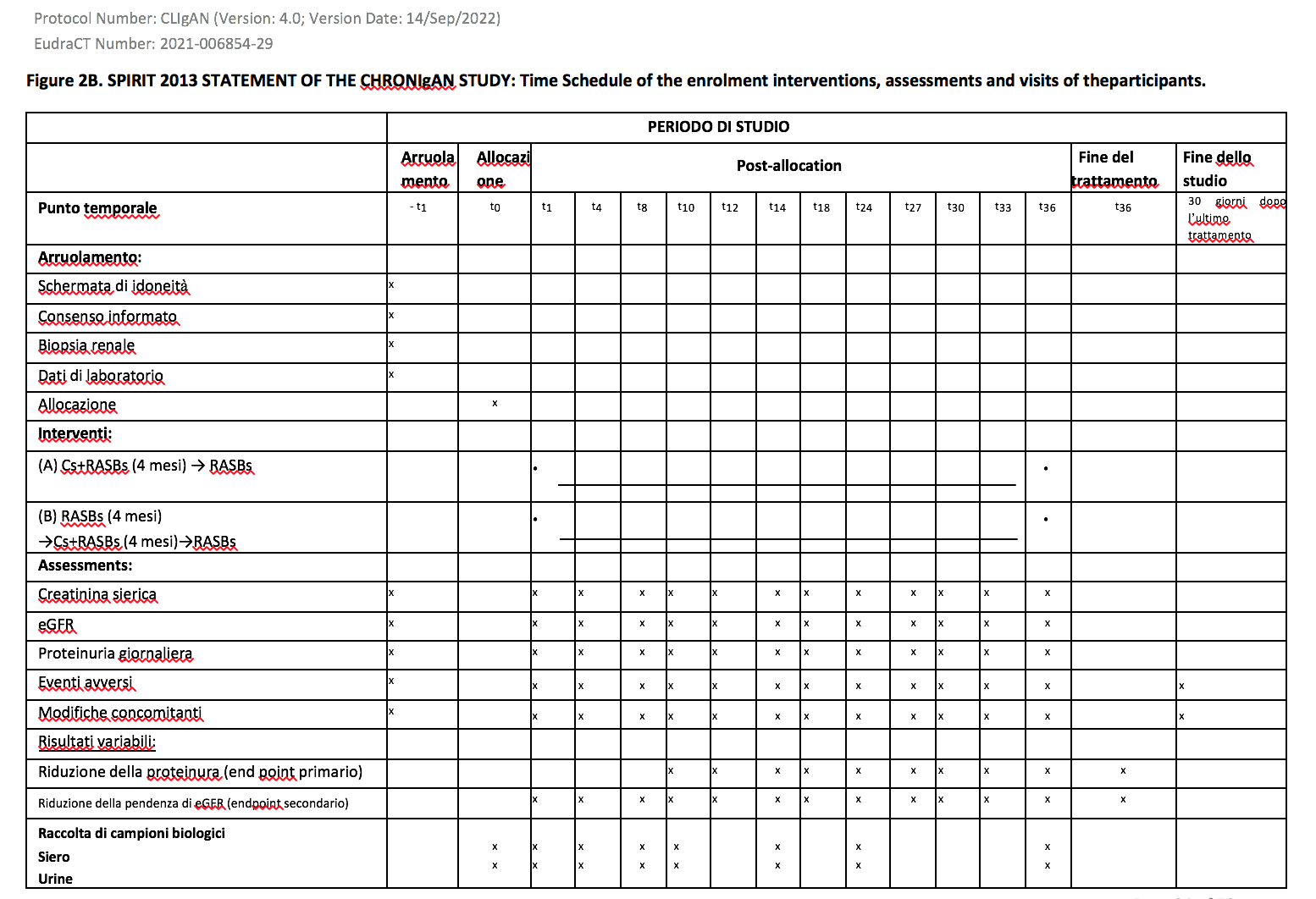
Laboratory tests during the follow-up The tests
that will be performed at each visit, as seen in the diagrams
attached to the protocol (Figures 2A and 2B), can be carried out in
the hospital laboratory or in a laboratory that has an agreement
with the National Health Service, as long as it is always the same
to allow a better comparison of the values when the patient comes to
visit. The ideal methodological approach is to carry out the
laboratory tests in the hospital where the patient receives the
outpatient visit, at least in the first 6 - 12 months after enrolment.
During hospitalization and follow-up, the following biological samples should be collected:
Blood. After the diagnosis of IgAN, carried
out with the kidney biopsy, take a blood sample of 10 ml to obtain 5
ml of serum. Store serum sample in refrigerator at -20ºC. The
blood sampling will be repeated at each outpatient visit. The date and patient code will be reported on the sample
Urine. After the diagnosis of IgAN, carried
out with the kidney biopsy, in the absence of gross haematuria, take a
20 ml sample from the 24-hour urine, suitably shaked to prevent urine
collection stratification. Store the urine tube in the refrigerator
at -20ºC.
At each outpatient visit a 24-hour urine sample will be collected by the patient who will have to shake the urine collected in the 24 hours before taking the sample. Only the patient's code, the date of the collection and the 24-hour urine quantity will be placed on the urine sample by the healthcare personnel.
In IgAN patients with active renal lesions (eGFR ≥ 30
ml/min/1.73 m2, proteinuria ≥ 0.5 g/24 hours) blood and urine samples will be taken at the time of enrollment (T0) and subsequently during the follow-up: T1, T2, T3, T4, T6, T8, T12, T24, T36. The number indicates the month post-enrollment.
In IgAN patients with chronic or moderate renal lesions (eGFR ≥30 ml/min/1.73 m2, proteinuria ≥0.5 g/24 hours) blood and urine samples will be taken at the time of enrollment (T0) and subsequently during the follow-up: T1, T4, T8, T10, T12, T24, T36. The number indicates the month post-enrollment.
In summary, at each outpatient visit, the patient must bring with him
a urine sample where the quantity of urine collected over 24 hours is reported; in addition, blood will be drawn, as indicated above.
NW. Please observe the following points for the purpose of the study:
- Explain in detail to the patient how the 24-hour urine collection is performed. Specify that the 24-hour urine collection container must be purchased only once. From the 24-hour urine, 20 ml will be taken and transferred to a test tube that will be taken to the outpatient visit. The patient code, the 24-hour urine amount and the collection date must be present on the test tube. The tube will be stored at -20ºC. At home, the patient will throw the remaining part of the urine into the toilet and then wash it. After washing with water only, the container must be kept for the next 24-hour urine collection to be carried out at each outpatient visit in the established months.
- Kindly provide the Reference Center (Prof. Schena) with information on the methods adopted by the hospital laboratory with the relative normal values of the following dosages: cretininemia, urea, proteinuria and albuminuria. These variables will be part of the primary endpoint. Creatininaemia and eGFR will be used for the secondary end point.