Protocol for processing kidney biopsy by light microscopy.
- Upon arrival of the sample, after evaluation of the adequacy and potential exploitation for the different study techniques, the material used for optical microscopy is fixed in neutral buffered formalin (NBF) at 10% for about 30-60 min (no longer of 24h).
- Once fixed, the bioptic tissue is placed in a biocassette with the patient's unique identification associated with a barcode.
- The biocassette containing the material is inserted into the automatic processor which exposes the sample to increasing concentration of alcohol (70%, 95% and absolute aclohol) with final exposure to xylene, with times set automatically by the processor. Alternatively, in the absence of an automatic processor, the sample is subjected manually to the alcohol scale as follows:
- DEHYDRATION
- Alcohol 50º 30' for renal tissue
- Alcohol 70º 30' for renal tissue
- Alcohol 95º 20' for renal tissue
- Alcohol 95º 20' for renal tissue
- Alcohol 100º 20' for renal tissue
- Alcohol 100º 20' for renal tissue
- CLARIFICATION
- Xylene º 20' for renal tissue
- At the end of the processing cycle, the tissue is infiltrated with paraffin (1h 30ºC) and included in a block with the same identification code present on the biocassette.
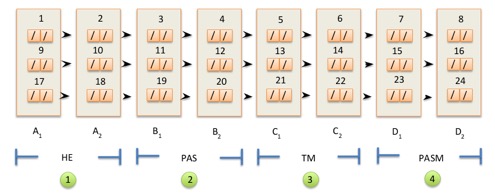
- Once ready, the block is subjected to microtome cutting to obtain sections (2-3 micron thick) that will be used for the Hematoxylin Eosin stains, silver impregnation acc. Jones, Periodic Acid Schiff (PAS), and Masson's Trichrome as shown in Figure 1. Specifically, slides designated as A2, B2, C2, and D2 will be used for peer review process in the CLIgAN study.
- In detail, the used protocol provides consecutive sections of the tissue placed on slide N.1 starting from the region closest to the label with progressive numbering of the slides which follows the descending trend of the tissue levels, as illustrated in Figure 1. In presence of small amount of renal tissue, only two sections per slide can be applied.
- Between one row and the next, two sections are kept on a loaded slide for any additional immunohistochemical stainings. In case the fresh renal tissue sample used for immunofluorescence has not reached the laboratory or is inadequate due to the absence of glomeruli, where possible 8 additional "blank" sections are loaded on a polylysinie slide between one row and the next for the execution of immunofluorescence obtained from paraffin renal tissue.
- Following the cutting phase, the slides obtained are subjected to deparaffinization as follows:
- DEPARAFINING and HYDRATION
- Xylene 4 min
- Alcohol 100º 2 min
- Alcohol 95º 2 min
- Alcohol 70º 2 min
- Alcohol 50º 2 min
- Washing in distilled water

Protocol for staining specimens used for optical microscopy.
- Hematoxylin and Eosin with automatic stainer
- Hematoxylin and Eosin - by hand protocol
- Slides already paraffinated will be inclusded in distilled water
- Hematoxylin:Dip the slides in the dye for 4 min
- Drain under spring water
- Alcohol- Acid: Drop with dropper on slide (recovering in the "mother" solution)
- Immediately dab in distilled water
- Quick wash in distilled water
- Eosin:Dip the slides in the dye for 5 min
- Quick wash in distilled water
- Dehydrate and assemble
- Alcohol 95º 1 min
- Alcohol 100º 2 min
- Xylene 5 min
- Mount the slides with Eukitt
- Hematoxylin: Mayer's haemallum (Bio-Optica)
- Eosin (0.5%): 0.5 gr Eosin in 100 ml of solution add 0.5 ml of glacial acetic acid
- Alcohol-acid : 100 ml Alchool 70% + 0.5 ml HCl conc. 37%
The Tissue-Tek Prisma automated stainer with automated slide mounter Tissue-Tek Film Coverslipper (Sakura) is used for Hematoxylin Eosin (EE). In the instrument there are 24 trays with a capacity of 650ml containing the following reagents:

The instrument is started, the basket containing the kidney biopsy slides is placed in one of the three input trays, the staining program is selected and at the end of the process the basket is moved to the coverslipper compartment for automatic film application.
Staining: nuclei dark blue, cytoplasm light pink. Study of inflammatory infiltrates, tubular cytoplasm and glomerular fibrinoid deposits.
Solutions:
Protocol for staining
specimens used for optical microscopy.
- Silver impregnation according to Jones
- Dip the sections in distilled water.
- Place 10 drops of reagent A on the section: leave to act for 30 minutes.
- Wash in distilled water.
- Set up the humidity chamber and place the slide with the sections facing up. Spill 10 drops of reagent B into the small capsule attached to the package, add 10 drops of reagent C and 10 drops of reagent D, shake and place the obtained solution on the section: close the humid chamber and incubate in an oven at 60ºC. Leave on for 30-40 minutes.
- Remove the humid chamber from the oven, open the cover and check the tone of the impregnation: if the blackening is correct, leave the slide to cool for 5 minutes and wash it in distilled water. If it is not sufficient, incubate it again in the oven and check every 5 minutes .
- Place 10 drops of E reagent on the section: leave to act for 1 minute.
- Wash in distilled water.
- Place 10 drops of F reagent on the section: leave to act for 1 minute.
- Wash in distilled water
- Dehydrate through the ascending series of alcohols, xylene and balsam.
- At the end of the staining, the section is counterstained with Hematoxylin Eosin inside the automatic stainer for about 30 minutes.
- Periodic acid solution 30 ml
- Silver nitrate solution 30 ml
- Hexamethylenetetramine solution 30 ml
- Sodium tetraborate solution 30 ml
- Gold chloride solution 30 ml
- Fixing solution 30 ml
- Silver impregnation according to Jones (protocol without BioOptica kit)
- Slides already deparaffinized and included in distilled water..
- Periodic acid (1%) ................................ Dip the slides for 25 min
- Quick washing in distilled water
- Working Xexamine silver solution ..................... Dip the slides for 1 hour (check under the microscope every 2 minutes)
- Quick wash in distilled water.
- Gold chloride (0.2%) ................................ differentiate in 2-3 steps
- Quick wash in distilled water.
- Sodium thiosulfate (2%) ................................ Dip the slides for 1 min
- Bouin ................................ Dip the slides at 60ºC for 30 min or over night at room temp.
- Quick wash in spring water: until all sections are white
- Quick wash in distilled water
- A.C Phosphotungstico (1%) ................................ Dip the slides for 1 - 2 min
- Quick wash in distilled water.
- Chromotrope 2R ................................ Dip the slides for 15 min
- Quick wash in distilled water.
- Dehydrate and assemble.
- Schiff's Periodic Acid (PAS)
- Dip the section in distilled water.
- Place 10 drops of A reagent on the tissue section: leave to act for 10 minutes.
- Wash in distilled water.
- Place 10 drops of B reagent on the tissue section: leave to act for 20 minutes.
- Wash in distilled water.
- Place 10 drops of C solution on the tissue section: leave to act for 2 minutes.
- Drain the slide and, without washing, place 10 drops of D reagent on the tissue section: leave to act for 2 minutes.
- Wash in distilled water.
- Put 10 drops of E reagent on the tissue section: leave to act for 3 minutes.
- Steep in running tap water for 5 minutes.
- Dehydrate in the ascending series of alcohols, xylene and balsam
- Periodic acid solution 30 ml
- Schiff's reagent 30 ml
- Potassium metabisulphite solution 30 ml
- Meyer's haemallum 30 ml
- Periodic Acid Schiff (PAS) without BioOptica kit
- Slides already deparaffinized and in distilled water.
- Periodic acid (1%) ................................ Dip the slides for 1 min
- Quick wash in spring water
- 2Quick washes in distilled water.
- Schiff's reagent ................................ Dip the slides for 25 min
- Quick wash in spring water; rinse until the water runs clear
- Quick wash in distilled water.
- Hematoxylin ................................ Dip the slides for 5 min
- Quick wash in spring water; rinse until the water runs clear
- Quick wash in distilled water.
- Alcohol - Acid: ................................ Drop with dropper on slide (recovering in the "mother" solution)
- Immediately dab in distilled water.
- Quick wash in distilled water.
- Dehydrate and assemble
- Alcohol 95º ................................ 1 min
- Alcohol 100º .............................. 2 min
- Xylene .............................. 5 min
- Mount the slides with Eukitt
- Periodic acid (1%) = 1ml in 100 ml of distilled water.
- Schiff's reagent: ready Bio-Optica (preserved in a cold room at +4ºC)
- Trichrome according to Masson
- Bring the section in distilled water.
- Place 6 drops of A reagent on the section, add 6 drops of B reagent: leave to act for 10 minutes.
- Without washing, drain the slide and place 10 drops of C solution on the section: leave to act for 4 minutes.
- Wash quickly (3-4 seconds) in distilled water and place 10 drops of D solution on the slide: leave to act for 4 minutes.
- Wash in distilled water and place 10 drops of E solution on the section: leave to act for 10 minutes.
- Without washing, drain the slide and place 10 drops of F solution on it: leave to act for 5 minutes.
- Wash in distilled water and rapidly dehydrate through the ascending series of alcohols, leaving 1 minute in the last steps: xylene and balsam.
- Ferric hematoxylin according to Weigert, A solution 18 ml
- Ferric hematoxylin according to Weigert, B solution 18 ml
- Picric acid, stabilized alcoholic solution 30 ml
- Ponceau B solution 30 ml
- Phosphomolybdic acid stabilized solution 30 ml
- Light green according to Goldner 30 ml
- Trichrome according to Masson without BioOptica kit
- Slides already deparaffinized and dip in distilled water.
- Fix it back in BOUIN ................................ 1-2h at 60ºC and/or all night at room temp.
- Quick wash in spring water; until all sections are white
- Quick wash in distilled water.
- Weigert's ferric hematoxylin ................................ 5-6 min
- Download in the Kartell box
- Quick wash in water dist.
- 10 drops of picric acid stabilized alcoholic solution
- Quick wash in distilled water.
- Ponceau fuchsin ................................ Dip the slides for 15 min
- 10 drops of Phosphomolybdic Acid (2%).
- Drain the slide without washing it.
- Light Green (2%) ................................ Dip the slide for 2 -3 min
- Washing in distilled H2O.
- Dehydrate and whip (immediately transfer to alcohol 95º)
- Alcohol 100º .............................. 2 min
- Xylene .............................. 5 min
- Mount the slides with Eukitt
- Picric acid in alcoholic solution .............................. 15 ml /50 ml.
- Formaldehyde .............................. 6 ml /20 ml.
- Glacial Acetic Acid .............................. 1.5 ml /5 ml.
- Picric acid in alcoholic solution: Alcohol 95º .............................. 758 ml.
- distilled water .............................. 142 ml.
To perform staining according to Jones steps the modified protocol of the kit supplied by Bio-Optica (n° 04-043822) is used. In detail, the method provides the following steps:
Method
Reactive
Protocol for staining
specimens used for optical microscopy
The modified protocol of the kit supplied by Bio-Optica is used to perform PAS staining(nº04-130802). In detail, the method provides the following steps:
Method
Reactive
Coloring:
Nuclei dark blue, cytoplasms pale pink, basement membranes, mesangial matrix and hyaline substance purple-red. Study of glomerular and tubular basement membranes and mesangial matrix to highlight glomerular sclerosis, arteriolar hyalinosis, and protein reabsorption droplets in glomerular and tubular epithelial cells.
Solutions:
Protocol for staining
specimens used for optical microscopy
To perform the Trichrome staining, the modified protocol of the kit supplied by Bio-Optica is used (nº 04-011802). In detail, the method provides the following steps:
Method
Reactive
Coloring:
Nuclei dark blue, cytoplasm pink, connective tissue and interstitium green color. Study of the glomeruli, tubules, interstitial space and basement membranes and its specific for the connective tissue. Provides information on the presence of inflammatory infiltrates and areas of interstitial fibrosis.
Solutions:
Bouin :